Intermolecular and very strong intramolecular C–Se⋯O/N chalcogen bonds in nitrophenyl selenocyanate crystals†
Abstract
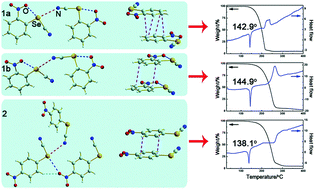
Single-crystal X-ray diffraction reveals that polymorphic ortho-nitrophenyl selenocyanate (o-NSC, crystals 1a and 1b) and monomorphic para-nitrophenyl selenocyanate (p-NSC, crystal 2) crystals are all stabilized mainly by intermolecular and very strong intramolecular C–Se⋯O/N chalcogen bonds, as well as by other different interactions. Thermogravimetric (TG) and differential scanning calorimetry thermogram (DSC) analyses show that the starting decomposition temperatures and melting points of the three crystals are different, following the order 1b > 1a > 2, which is consistent with the structural characteristics of the crystals. In addition, atoms in molecules (AIM) and natural bond orbital (NBO) analyses indicate that the total strengths of the C–Se⋯O and C–Se⋯N chalcogen bonds decrease in the order 1b > 1a > 2. This study could be significant for engineering functional crystals based on robust C–Se⋯O and C–Se⋯N chalcogen bonds, and for designing drugs containing selenium as well as understanding their interaction in biosystems.


 Please wait while we load your content...
Please wait while we load your content...