Interaction of gas phase copper(ii) acetylacetonate with slow electrons†
Abstract
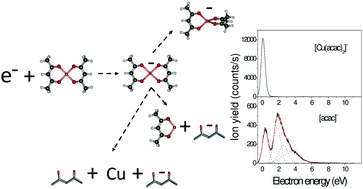
Understanding the fundamental processes underlying the interaction of organometallic compounds with low energy electrons is desirable for optimizing methodologies for nanoscale applications. In this work, we couple experimental measurements with theories to investigate the interaction of gas phase copper(II) acetylacetonate, Cu(acac)2, with low energy (<12 eV) electrons. Near 0 eV, a multipole-bound anion is likely to act as the doorway for the formation of a transitory molecular anion which then undergoes stabilization via a 90°-rotation of one of the acac units. The production of the parent anion competes with the dissociation processes, generating preferentially the acetylacetonate negative ion. Moreover, at incident electron energies above 3.5 eV, the electron driven fragmentation of Cu(acac)2 is likely to produce atomic Cu. These results can suggest some potential strategies for the deposition of pure copper using an appropriate electron irradiation technique.


 Please wait while we load your content...
Please wait while we load your content...