Aerogen bonds formed between AeOF2 (Ae = Kr, Xe) and diazines: comparisons between σ-hole and π-hole complexes†
Abstract
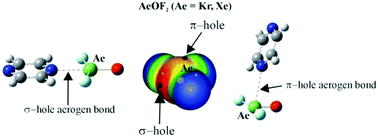
The interaction between KrOF2 or XeOF2 and the 1,2, 1,3, and 1,4 diazines is characterized chiefly by a Kr/Xe⋯N aerogen bond, as deduced from ab initio calculations. The most stable dimers take advantage of the σ-hole on the aerogen atom, wherein the two molecules lie in the same plane. The interaction is quite strong, as much as 18 kcal mol−1. A second class of dimer geometry utilizes the π-hole above the aerogen atom in an approximate perpendicular arrangement of the two monomers; these structures are not as strongly bound: 6–8 kcal mol−1. Both sorts of dimers contain auxiliary CH⋯F H-bonds which contribute to their stability, but even with their removal, the aerogen bond energy remains as high as 14 kcal mol−1. The nature and strength of each specific interaction is confirmed and quantified by AIM, NCI, NBO, and electron density shift patterns. There is not a great deal of sensitivity to the identity of either the aerogen atom or the position of the two N atoms in the diazine.
- This article is part of the themed collections: 1st International Conference on Noncovalent Interactions and 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...