Reactivity of hydropersulfides toward the hydroxyl radical unraveled: disulfide bond cleavage, hydrogen atom transfer, and proton-coupled electron transfer†
Abstract
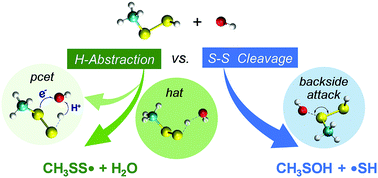
Hydropersulfides (RSSH) are highly reactive as nucleophiles and hydrogen atom transfer reagents. These chemical properties are believed to be key for them to act as antioxidants in cells. The reaction involving the radical species and the disulfide bond (S–S) in RSSH, a known redox-active group, however, has been scarcely studied, resulting in an incomplete understanding of the chemical nature of RSSH. We have performed a high-level theoretical investigation on the reactions of the hydroxyl radical (˙OH) toward a set of RSSH (R = –H, –CH3, –NH2, –C(O)OH, –CN, and –NO2). The results show that S–S cleavage and H-atom abstraction are the two competing channels. The electron inductive effect of R induces selective ˙OH substitution at one sulfur atom upon S–S cleavage, forming RSOH and ˙SH for the electron donating groups (EDGs), whereas producing HSOH and ˙SR for the electron withdrawing groups (EWGs). The H-Atom abstraction by ˙OH follows a classical hydrogen atom transfer (hat) mechanism, producing RSS˙ and H2O. Surprisingly, a proton-coupled electron transfer (pcet) process also occurs for R being an EDG. Although for RSSH having EWGs hat is the leading channel, S–S cleavage can be competitive or even dominant for the EDGs. The overall reactivity of RSSH toward ˙OH attack is greatly enhanced with the presence of an EDG, with CH3SSH being the most reactive species found in this study (overall rate constant: 4.55 × 1012 M−1 s−1). Our results highlight the complexity in RSSH reaction chemistry, the extent of which is closely modulated by the inductive effect of the substituents in the case of the oxidation by hydroxyl radicals.


 Please wait while we load your content...
Please wait while we load your content...