BaCO3 and NH3SO3 as precursors for the hydrothermal synthesis of BaSO4†
Abstract
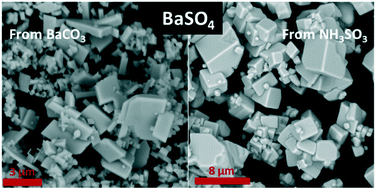
In this study, we demonstrated that BaCO3 and NH3SO3 can be conveniently used as BaSO4 precursors. BaSO4 is obtained by treating the precursor, BaCO3, with a sulfate-containing solution, owing to the lower solubility product constant of BaSO4 compared to that of BaCO3. Using this procedure, BaSO4 particles with pseudo-cubic/prismatic morphology are obtained. In this case, temperature and reaction time have little influence on morphology, which is mainly controlled by supersaturation and sulfate concentration. Sulfate counter ion also affects the morphology, but its effect is less pronounced. The hydrolysis of NH3SO3 in the presence of soluble barium salts produces BaSO4 particles, and different kinds of morphology can be obtained, encompassing rounded and nearly spherical particles, prismatic and pseudo-cubic, irregular shapes with flat and well-developed facets, and even hopper crystals. In these syntheses, temperature and pH are playing a major role in controlling sulfamic acid hydrolysis and, therefore, supersaturation, with remarkable consequences for crystal nucleation and growth. Considering that BaSO4 finds applications in many sectors and for different purposes, the variety of particle size and shape reported in this study can potentially improve the performance of the materials in which BaSO4 particles are employed.


 Please wait while we load your content...
Please wait while we load your content...