A novel red phosphor of Mn4+ ion-doped oxyfluoroniobate BaNbOF5 for warm WLED applications
Abstract
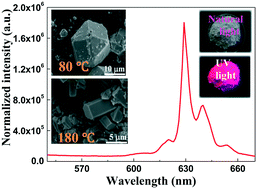
A red phosphor composed of Mn4+-activated oxyfluoroniobate BaNbOF5 was synthesized from the starting materials BaF2, Nb2O5, and K2MnF6 in an HF solution by using a method based on dissolution ion-exchange recrystallization. The BNOF:Mn phosphor particles transformed from micron-sized polyhedrons to rectangular blocks as the reaction temperature was increased from room temperature to 180 °C. The effects of the synthetic parameters, such as the concentration of K2MnF6 in the reaction system and the reaction temperature, on the luminescence intensity of BNOF:Mn were investigated by acquiring detailed experimental results. The as-prepared BNOF:Mn phosphor displayed a broad and intense absorption in the blue-light region and a bright luminescence in the red-light region with a color purity of 97.7% at the Commission Internationale de l'Eclairage (CIE) chromaticity coordinates of x = 0.594, y = 0.390. The BNOF:Mn phosphor showed superior thermal stability and its activation energy Ea was determined to be 0.506 eV. The advantage of the synthetic strategy to obtain BNOF:Mn can be extended to developing oxyfluoroniobates based on Mn4+-doped red phosphors, which might have the advantages of both oxides and fluorides.


 Please wait while we load your content...
Please wait while we load your content...