Phase and morphology transformation from assembled hexagonal HTiOF3 prisms to {001} faceted TiO2 nanosheets
Abstract
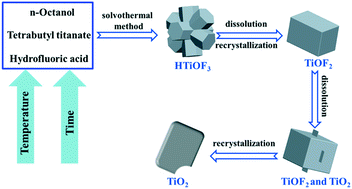
A three-stage synthesis method was developed for the preparation of TiO2 nanosheets with exposed {001} facets by a solvothermal process. The formation mechanism of the TiO2 nanosheets was investigated by the variation of the solvothermal temperature and reaction time. In the three-stage synthesis process, assembled hexagonal HTiOF3 prisms were produced in the first stage, and then the hexagonal HTiOF3 prisms transformed gradually into cubic TiOF2 in the second stage. Anatase TiO2 nanosheets with exposed {001} facets are formed from cubic TiOF2 after the third stage. The results reveal that the reaction time and temperature play important roles in controlling the transformation of the morphology and phase from the assembled hexagonal HTiOF3 prisms into the TiO2 nanosheets through cubic TiOF2. In addition, compared with hexagonal TiO2 prisms and cubic TiO2 calcined from HTiOF3 and TiOF2, the TiO2 nanosheets synthesized by the three-stage solvothermal strategy exhibited better photocatalytic performance. The finding of this work presents a distinctive formation mechanism of TiO2via intermediates HTiOF3 and TiOF2 to obtain TiO2 nanosheets with controllable morphologies and excellent properties.


 Please wait while we load your content...
Please wait while we load your content...