Two-component molecular crystals: relationship between the entropy term and the molecular volume of co-crystal formation†
Abstract
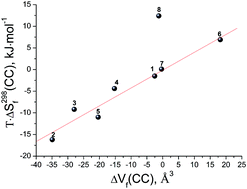
There are very few articles that investigate the thermodynamic formation of two-component molecular crystals. For this reason, it is difficult to conduct a statistically meaningful comparative analysis of experimentally obtained data of the entropy terms and molecular volumes of co-crystal formation. In order to realize this analysis, the entropy term was taken both from experimental data presented in the literature and from theoretical calculations by the approach we developed earlier. Linear dependences between the parameters in question have been found, which makes it possible to evaluate the entropy term of co-crystal formation knowing only the crystalline structure of the co-crystal and the individual components constituting it.


 Please wait while we load your content...
Please wait while we load your content...