What is responsible for conformational diversity in single-crystal tetraoxazaspiroalkanes? X-Ray, DFT, and AIM approaches†
Abstract
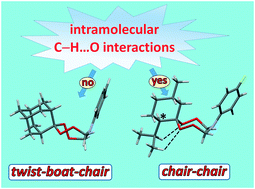
The conformational mobility of large heteroatomic cycles (>6) in organic compounds studied in the liquid state is a major challenge. This is due to low energy barriers between the conformational isomers (1–2 kcal mol−1), which make it difficult to find the most important factors in their preference. Eleven single crystal tetraoxazaspirocycloalkanes are considered in this work with single-crystal X-ray diffraction, DFT, and AIM calculations. Natural bond orbital analysis at the B3LYP/6-31G(d,2p) level of theory and topological analysis of the electron density within Bader's theory of “Atoms in Molecules” showed that the tetraoxazocane cycle in spiro-adamantanetetraoxazocanes and tetraoxazaspiroalkanes possesses a twist–boat–chair conformation due to the lower interaction energy of LP(O6) → σ*(C5–N4) and the large contribution of the π-component to the C3–O2 bond. The tetraoxazocane cycle in isopropyl-methyl-tetraoxaza-spiro-tridecanes adopts a chair–chair conformation due to the higher interaction energy of LP(O6) → σ*(C5–N4) and the smaller contribution of the π-component. A key factor, which determines the large O1–O2–O6–O7 pseudo-torsion angles (102–112°) in spiro-adamantanetetraoxazocanes and tetraoxaza-spiro-alkanes, is the absence of intramolecular C–H⋯O and C–H⋯π contacts and vice versa, which provide smaller O1–O2–O6–O7 pseudo-torsion angles (0.6–4°) in isopropyl-methyl-tetraoxaza-spiro-tridecanes. The molecules of spiro-adamantanetetraoxazocanes and tetraoxazaspiroalkanes in single crystals are stabilized via C–H⋯O and C–H⋯H–C weak intermolecular interactions. The molecules of isopropyl-methyl-tetraoxaza-spiro-tridecanes are packed in crystals with C–H⋯O unusual bifurcated one-component and multicomponent contacts.


 Please wait while we load your content...
Please wait while we load your content...