Crystallization kinetics of Na2CO3 in Bayer aluminate solutions during the evaporation process
Abstract
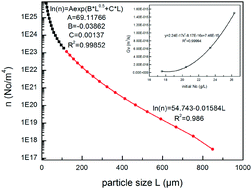
The inevitable Na2CO3 in caustic aluminate solution is the major component of scale formed in the evaporation process. A deeper understanding of the Na2CO3 crystallization kinetics in Bayer aluminate solutions during the evaporation process is necessary to further understand the scaling process. The kinetics of Na2CO3 crystallization in sodium aluminate solutions during the evaporation process were investigated in a continuously operated mixed suspension mixed product removal (MSMPR) crystallizer. Based on the particle size distribution (PSD) of crystals in the MSMPR crystallizer, a typical population density distribution curve of Na2CO3 crystals was obtained. Furthermore, an agglomeration population balance model was used to calculate the kinetic parameters through the population density distribution. When the initial content of Na2CO3 (Nc) is in the range of 17.54–26.32 g L−1, the agglomeration kernel has a good linear relationship with the initial Nc, while the volume growth rate and the nucleation rate have an obvious quadratic polynomial relationship with the initial Nc. All the regressions of the parameters had an adjusted R squared higher than 0.9945.


 Please wait while we load your content...
Please wait while we load your content...