Hydrothermal synthesis of a rutile/anatase TiO2 mixed crystal from potassium titanyl oxalate: crystal structure and formation mechanism
Abstract
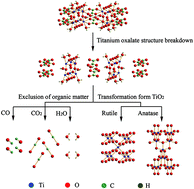
Nowadays, rutile TiO2 or anatase TiO2 is prepared from titanium oxalate, but there are only few studies on the rutile/anatase TiO2 mixed crystal. In this study, using potassium titanyl oxalate as the titanium source, titanium oxalate [Ti2O3(H2O)2(C2O4)·H2O] has been prepared by a one-step hydrothermal method at 120 °C. When the temperature was increased to 150 °C, the durian-shaped rutile/anatase TiO2 mixed crystal was obtained, and the content of the anatase phase was about 42.5%. The products were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), thermal gravimetric analysis (TG) and differential thermal analysis (DTA). The crystal structures of the products were simulated, and the formation mechanism of the products was discussed. Photocurrent densities of 19.47 μA cm−2 were obtained under illumination of rutile/anatase TiO2 mixed crystal photoelectrodes. The nanostructure of the rutile/anatase TiO2 mixed crystal is formed by epitaxial growth of titanium oxalate along specific surfaces, and all crystal structures and connection modes of TiO6 octahedron are similar.


 Please wait while we load your content...
Please wait while we load your content...