Tetrahalometallate salts of N-(4-picolinium)-1,8-naphthalimide: structures and solid-state fluorescence†
Abstract
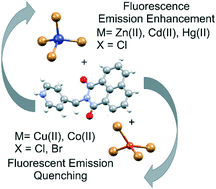
The structural characteristics and solid-state fluorescence behaviour of organic–inorganic hybrid compounds formed through the combination of the N-(4-picolinium)-1,8-naphthalimide (pnH) cationic fluorophore with tetrahalometallate anions were investigated. Eight novel crystal structures, of the formula 2(C18H13N2O2)+[MX4]2−, were obtained through the combination of divalent metal halide salts and pnH cations, with [MX4]2− = [CoCl4]2− in structure pnHCoCl, [CoBr4]2− in pnHCoBr, [ZnCl4]2− in pnHZnCl, [ZnBr4]2− in pnHZnBr, [CdCl4]2− in pnHCdCl, [HgCl4]2− in pnHHgCl, [CuCl4]2− in pnHCuCl and [CuBr4]2− in pnHCuBr. To highlight the effect of halide salt formation, the structures and fluorescence behaviour of N-(4-picolinium)-1,8-naphthalimide chloride, pnHCl, and N-(4-picolinium)-1,8-naphthalimide bromide, pnHBr, were also studied. The effect of the formation of the halide- or tetrahalometallate salt on the solid-state fluorescence properties relative to that of N-(4-picolyl)-1,8-naphthalimide (pn) is highlighted. In addition, packing trends across the family and crystal engineering synthons are identified. It is illustrated that the fluorescence emission of the pn fluorophore can be tweaked through the formation of its halide- or tetrahalometallate salt.


 Please wait while we load your content...
Please wait while we load your content...