Why lamivudine assembles into double-stranded helices in crystals: salt heterosynthon versus base-pairing homosynthon†
Abstract
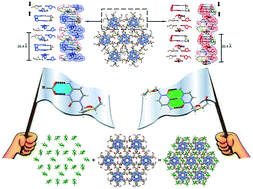
Here we were interested in obtaining a better understanding of the competition between the salt heterosynthon and the base-pairing homosynthon formed by the anti-HIV drug lamivudine in the presence of strong acids. Even though the preparation of the multicomponent crystal forms of this drug with weak or moderate acids is well investigated, the crystallization of lamivudine with strong acids is still little explored. Besides filling this crystallization screening gap, the driving forces of the so-called lamivudine duplexes could be drawn from the complete structural landscape and the theoretical thermodynamic parameters of the salt heterosynthon, calculated at the M06-2X/6-31+G** level of theory. Five new crystal structures of lamivudine were obtained, wherein two of them were assembled as base-paired helically-stacked strands in the presence of trifluoroacetic and trichloroacetic acids (lamivudine duplexes V and VI, respectively). Besides, three salts were prepared by crystallization of lamivudine with sulfuric and perchloric acids. Finally, the theoretical approach showed that there is no energy trend regarding the formation of lamivudine duplexes with aliphatic organic acids or lamivudine salts with aromatic acids, which is usually observed in practice.


 Please wait while we load your content...
Please wait while we load your content...