3-Homoacyl coumarin: an all carbon 1,3-dipole for enantioselective concerted (3+2) cycloaddition†
Abstract
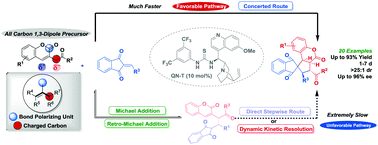
A new type of all-carbon 1,3-dipole precursor, 3-homoacylcoumarin, was employed for the stereoselective (3+2) cycloaddition with indandione alkylidenes to generate a series of coumarin/indandione-fused spirocyclopentanes bearing four contiguous stereogenic centers. While two reaction pathways progressed simultaneously, detailed mechanistic investigation revealed that the highly efficient stereoselective concerted route dominated the extremely slow stepwise pathway.


 Please wait while we load your content...
Please wait while we load your content...