Efficient and regioselective synthesis of γ-lactone glycosides through a novel debenzylative cyclization reaction†
Abstract
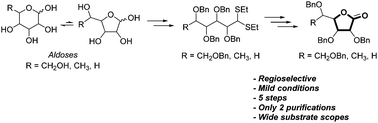
An efficient and regioselective approach for the construction of synthetically important γ-lactone glycosides is reported from unprotected aldoses through a new debenzylative lactonization (DBL) reaction. The scope and limitations of this DBL reaction are described starting from a series of commercially available hexoses (L-fucose, D-galactose, D-glucose) and pentoses (D-arabinose, D-ribose, D-lyxose, D-xylose) to afford the corresponding γ-lactones in good yields and without concomitant δ-lactone formation.


 Please wait while we load your content...
Please wait while we load your content...