Abstract
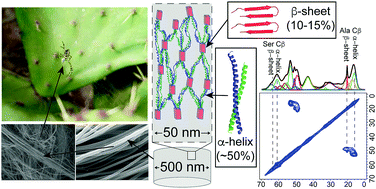
Solid-State NMR results on 13C-Ala/Ser and 13C-Val enriched Argiope argentata prey-wrapping silk show that native, freshly spun aciniform silk nanofibers are dominated by α-helical (∼50% total) and random-coil (∼35% total) secondary structures, with minor β-sheet nanocrystalline domains (∼15% total). This is the most in-depth study to date characterizing the protein structural conformation of the toughest natural biopolymer: aciniform prey-wrapping silks.


 Please wait while we load your content...
Please wait while we load your content...
