Chemoselective isocyanide insertion into the N–H bond using iodine–DMSO: metal-free access to substituted ureas†
Abstract
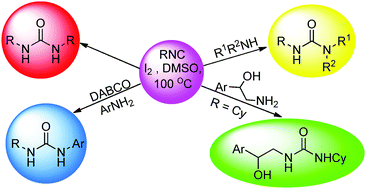
Insertion of isocyanides into the N–H bond gives access to many medicinally important and structurally diverse complex nitrogen-containing heterocycles. Although the transition metal catalyzed isocyanide insertion into the N–H bond is very common, polymerization of isocyanides in the presence of a transition metal and their strong coordination with metals are the common drawbacks. On the other hand, the inertness of most of the isocyanides towards amines in the absence of a metal catalyst has stymied the growth of the metal-free approach for isocyanide insertion into amines. As a result, only a handful of metal catalysed methods with limited substrate scopes have been reported for the synthesis of ureas via isocyanide insertion into amines and no metal-free version has been reported yet. Interestingly, chemoselective isocyanide insertion into amines has not been reported in the literature. We employed the I2–DMSO reagent system for the chemoselective synthesis of ureas, where isocyanides react with aliphatic amines only, while aromatic amines need a nucleophilic activator (DABCO) to facilitate the formation of ureas. This method gave direct and chemoselective access to ureas by evading the commonly used yet toxic isocyanates.


 Please wait while we load your content...
Please wait while we load your content...