Reversible 1,1-hydroaluminations and C–H activation in reactions of a cyclic (alkyl)(amino) carbene with alane†
Abstract
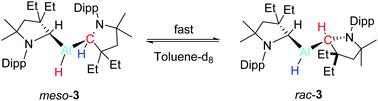
Varying the reaction ratio of cyclic (alkyl)(amino) carbene (cAACEt) with AlH3·NEtMe2 leads to the isolation of (cAACEtH)AlH2·NEtMe21 and (cAACEtH)2Al(μ-H)2AlH2·NEtMe22 and the first example of a monomeric dialkyl-aluminum hydride (cAACEtH)2AlH 3. VT and 1H–1H EXSY NMR experiments of 3 demonstrated the isomerization of the diastereomers of 3via the first instance of reversible hydride migration between the Al and the C center. In addition, heating solutions of 3 at 100 °C affords (cAACEtH)Al(CHC(Et)2CH2C(Me)2NC6H3(iPr)C(Me)CH2) 4 with loss of H2.


 Please wait while we load your content...
Please wait while we load your content...