Asymmetric synthesis of tetrahydroisoquinoline-fused spirooxindoles as Ras-GTP inhibitors that inhibit colon adenocarcinoma cell proliferation and invasion†
Abstract
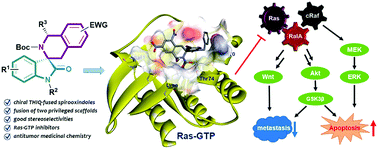
Here we report the synthesis of a library of chiral THIQ-fused spirooxindoles, which combine two privileged scaffolds in antitumor medicinal chemistry. Some of the library members inhibit the proliferation and invasion ability of Ras-mutated colon adenocarcinoma cells. Mechanistic studies suggest that the most potent compound, 3m, inhibits Ras-GTP and thereby suppresses downstream signaling mediated by MAPK, PI3K-Akt and Wnt. Ultimately this leads to mitochondrial apoptosis.


 Please wait while we load your content...
Please wait while we load your content...