Cu(ii)–tBu–PHOX catalyzed enantioselective malonate addition onto 3-hydroxy 2-oxindoles: application in the synthesis of dimeric pyrroloindoline alkaloids†
Abstract
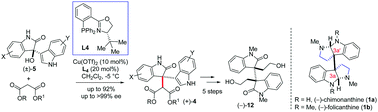
An efficient Cu(II)–PHOX-catalyzed malonate addition onto 3-hydroxy 3-indolyl-2-oxindoles is envisioned to afford excellent enantioselectivities (up to >99% ee) in high chemical yields. Detailed characterization techniques including X-ray, NMR, CV and EPR experiments suggest that a Cu(II)-complex is involved as an active species in this process. Applying this strategy, an advanced intermediate of cyclotryptamine alkaloids has been synthesized in few steps for a general approach to bis-cyclotryptamine alkaloids.


 Please wait while we load your content...
Please wait while we load your content...