Zinc-catalyzed reaction of isoxazoles with thioynol ethers involving an unprecedented 1,2-sulfur migration†
Abstract
A novel zinc-catalyzed reaction of isoxazoles with thioynol ethers involving an unprecedented 1,2-sulfur migration has been developed, which represents the first example of a non-noble metal-catalyzed reaction between isoxazoles and alkynes. This method allows the facile and atom-economical synthesis of a range of valuable β-keto enamides. Moreover, the computational study provides further evidence for the feasibility of the proposed reaction mechanism.
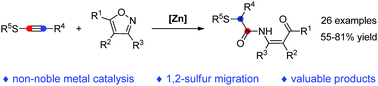


 Please wait while we load your content...
Please wait while we load your content...