Transition-metal-free insertion reactions of alkynes into the C–N σ-bonds of imides: synthesis of substituted enamides or chromones†
Abstract
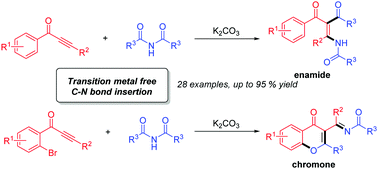
A transition-metal-free tandem process for the synthesis of substituted enamides and chromones is presented. The insertion of isolated alkynes into the C–N σ-bonds of imides is involved in this tandem process. In the case of alkynones bearing an ortho-bromo-substituted aryl ring, chromones were selectively formed via the O-cyclization pathway. A variety of substituted enamides and chromones were prepared in good to high yields.


 Please wait while we load your content...
Please wait while we load your content...