Kinetic barriers to α-synuclein protofilament formation and conversion into mature fibrils†
Abstract
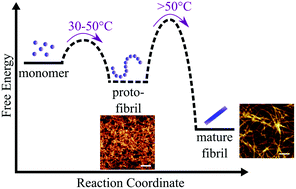
Oligomeric and protofibrillar aggregates that are populated along the pathway of amyloid fibril formation appear generally to be more toxic than the mature fibrillar state. In particular, α-synuclein, the protein associated with Parkinson's disease, forms kinetically trapped protofibrils in the presence of lipid vesicles. Here, we show that lipid-induced α-synuclein protofibrils can convert rapidly to mature fibrils at higher temperatures. Furthermore, we find that β-synuclein, generally considered less aggregation prone than α-synuclein, forms protofibrils at higher temperatures. These findings highlight the importance of energy barriers in controlling the de novo formation and conversion of amyloid fibrils.


 Please wait while we load your content...
Please wait while we load your content...