Alkene protection against acid using a bromide substituent: application in a total synthesis of (−)-6,7-dideoxysqualestatin H5†
Abstract
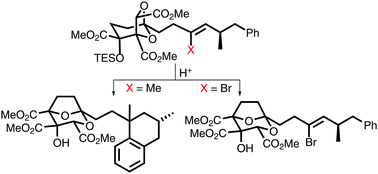
The presence of a bromide substituent, instead of a hydrogen or methyl group, on a carbon–carbon double bond, protects the alkene from addition reactions when exposed to trifluoroacetic acid. This concept is used to circumvent concomitant loss of unsaturation in a late-stage acid-catalysed 6,8- to 2,8-dioxabicyclo[3.2.1]octane rearrangement towards (−)-6,7-dideoxysqualestatin H5. The inertness of the alkenyl bromide functionality is demonstrated through several synthetic transformations in the assembly of the rearrangement substrate. Completion of the natural product synthesis is facilitated by post-rearrangement removal of the bromide substituent through stereoselective C–C cross-coupling in the presence of ester and hydroxyl functionalities.
- This article is part of the themed collection: Natural product synthesis


 Please wait while we load your content...
Please wait while we load your content...