A generalized approach for NMR studies of lipid–protein interactions based on sparse fluorination of acyl chains†
Abstract
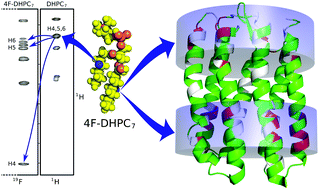
Sparse lipid fluorination enhances the lipids' 1H signal dispersion, enables clean molecular distinction by 19F NMR, and evinces micelle insertion of proteins via fluorine-induced signal shifts. We present a minimal fluorination scheme, and illustrate the concept on di-(4-fluoro)-heptanoylphosphatidylcholine micelles and solubilised seven-helix transmembrane pSRII protein.


 Please wait while we load your content...
Please wait while we load your content...