Rh-Catalyzed C–H bond alkylation of indoles with α,α-difluorovinyl tosylate via indolyl group migration†
Abstract
Herein we demonstrate that an alkylation of indoles could be accessed through C–H bond functionalization with α,α-difluorovinyl tosylate. The key aspect for the effective alkylation is the influence of the fluorine substituents on the reactivity of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond, allowing regioselective insertion as well as an indolyl group shift process. Furthermore, the fluorides are removed through alcoholysis to furnish the alkylation product as a traceless auxiliary.
C double bond, allowing regioselective insertion as well as an indolyl group shift process. Furthermore, the fluorides are removed through alcoholysis to furnish the alkylation product as a traceless auxiliary.
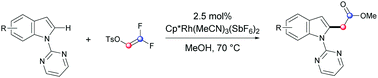


 Please wait while we load your content...
Please wait while we load your content...