Catechol oxidation: considerations in the design of wet adhesive materials†
Abstract
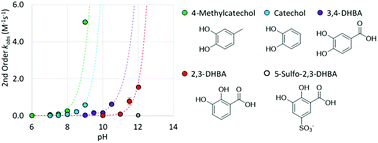
Catechols are found as functional groups in Nature as the 3,4-dihydroxy isomer, generally with electron donating substituents and as the 2,3-dihydroxy isomer, with an electron withdrawing substituent. Adhesive properties of catechol materials rely on the vicinal diol configuration, yet conversely, cohesive properties of catechol materials depend on catechol oxidation that promote subsequent crosslinking reactions. While the pH-dependent oxidation of catechol by dioxygen is well recognized, a better understanding of substituent effects on catechol redox chemistry is important in the design of new catechol-containing functional materials. The pH-dependent oxidation kinetics of catechol and substituted catechols by O2 was investigated with a Clark-type oxygen electrode. The results are consistent with a mechanism in which O2 oxidizes both mono-deprotonated and fully deprotonated catechol anions. A linear Hammett correlation for the pH-independent second order rate constants for catechol oxidation by O2 establishes that catechols functionalized with electron withdrawing groups have slower rates of oxidation by O2, whereas catechols with electron donating groups have faster rates of oxidation by O2. The Hammett correlation allows for selection of functionalized catechols with redox properties ideally suited for adhesive or crosslinking applications.
- This article is part of the themed collection: Celebrating our 2019 Prize and Award winners


 Please wait while we load your content...
Please wait while we load your content...