Rational functionalization of reduced graphene oxide with an imidazole group for the electrochemical sensing of bisphenol A – an endocrine disruptor†
Abstract
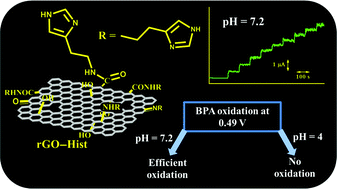
Reduced graphene oxide has been rationally functionalized with histamine for the highly sensitive and selective electrochemical determination of bisphenol A (BPA). Histamine is covalently attached to graphene oxide by amide coupling and the oxygen functionalities of graphene oxide are partially reduced electrochemically. The facilitated electrochemical oxidation of BPA was achieved in neutral pH with functionalized reduced graphene oxide. The pH of the reaction controls the oxidation of BPA. Electrochemical oxidation is not favourable at pH less than the pKa of histamine due to the protonation of the imidazole nitrogen. The electrode is highly sensitive (1727.29 ± 12.48 nA μM−1 cm−2) towards BPA and shows a linear response up to 30 μM of BPA. It could detect as low as 0.03 nM of BPA (S/N = 5). The other coexisting analytes do not interfere with the voltammetric measurement of BPA.


 Please wait while we load your content...
Please wait while we load your content...