A platinum oxide-based microvoltammetric pH electrode suitable for physiological investigations†
Abstract
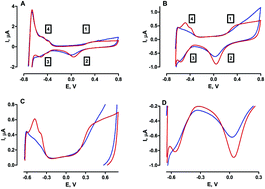
Attempts to develop miniaturised pH electrodes for in vivo monitoring have received much attention in recent years. Continuous real-time pH measurements may be predictive of potentially dangerous deviations in metabolic events that could improve patient prognosis. Herein, we report the in vitro investigation of a physiologically relevant, Pt oxide-based microvoltammetric pH electrode. Cycling through the potential window range −0.65 V to +0.8 V vs. SCE, gave rise to well-established monolayer oxide (MO) and hydrogen (H2) adsorption redox peaks in aqueous solution. The H2 desorption and MO reduction peaks demonstrated pH dependent, linear responses (49 ± 11 mV pH−1 and 76 ± 4 mV pH−1 respectively), following pre-activation of the electrode surface in HCl. Since in vivo monitoring is at the core of this design, the effect of incorporating a miniaturised pseudo reference electrode (PRE) was determined. The Ag/AgCl PRE demonstrated near Nernstian behaviour for the MO reduction peak (58 ± 5 mV pH−1) and sub-Nernstian behaviour (43 ± 6 mV pH−1) for its H2 desorption counterpart. Finally, a preliminary in vivo recording performed in the striatum of a freely moving mouse confirmed that the MO reduction peak was maintained under physiological conditions. These findings support the ability of the Pt oxide-based pH electrode to perform continuous, stable recordings in vivo and warrants further characterisation.


 Please wait while we load your content...
Please wait while we load your content...