The mechanism and conformational changes of polybrominated diphenyl ethers to TTR by fluorescence spectroscopy, molecular simulation, and quantum chemistry†
Abstract
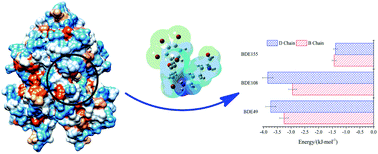
Extracellular deposition of transthyretin (TTR) as amorphous aggregates and amyloid fibrils is genetically and biochemically linked to a number of human diseases characterized by nervous system or organ dysfunction. The interaction mechanism of TTR with different polybrominated diphenyl ethers (PBDEs), such as BDE49, BDE108 and BDE155, was studied by a variety of spectroscopic techniques and computer simulations. The results of steady-state fluorescence, time-resolved fluorescence and UV-Vis spectroscopy showed that BDE49, BDE108 and BDE155 could cause fluorescence quenching of TTR, which was mainly static quenching and Förster's resonance energy transfer. Molecular docking and thermodynamic analysis further confirmed that the binding of PBDEs to TTR was mainly hydrophobic and formed a cation–π with the residue LYS15 in the TTR. Quantum chemistry and energy contribution analysis of the ligand and residue LYS15 revealed that the binding was mainly due to the cation–π formed by the C atom of the benzene ring and the polar residue LYS15 (NH3+) of TTR. Moreover, the energy contributions of BDE49, BDE108 and BDE155 to the residue LYS15 (B Chain and D Chain) are relatively large which enables their combination to be more stable. Therefore, the residue LYS15 in TTR plays a crucial role in various physiological activities in the human body.


 Please wait while we load your content...
Please wait while we load your content...