Arylene–vinylene terpyridine conjugates: highly sensitive, reusable and simple fluorescent probes for the detection of nitroaromatics†
Abstract
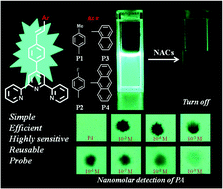
A series of highly emissive arylene–vinylene conjugated 4′-(4-{2-[aryl]-ethenyl}phenyl)-2,2′:6′,2′′-terpyridines (aryl = 4-methylphenyl, 4-fluorophenyl, 1-naphthyl, and 9-anthralyl in P1–P4 respectively) have been explored for the detection of nitroaromatic compounds (NACs). The synthesized push–pull fluorescent probes show remarkable sensitivity toward NACs in solution, vapor and contact mode as ‘turn-off’ sensors which can be visualized by the naked eye. The photophysical studies reveal that electron transfer occurs from the electron rich arylene–vinylene conjugated terpyridines to the electron-deficient NACs through supramolecular complexation which is further illustrated by the time-resolved fluorescence and 1H NMR titration experiment. The arylene–vinylene conjugated terpyridines offer excellent sensitivity toward picric acid (PA) exhibiting nanomolar detection in contact mode, and ppm level detection in the solution state with association constants in the range of 2.54–8.08 × 104 M−1. The HOMO–LUMO energy levels have been calculated from the electrochemical and TDDFT studies to understand the efficacy and the mechanism of electron transfer from the probe to the NACs leading to fluorescence quenching. The reversibility, recyclability and contact mode detection of PA at a nanomolar level demonstrates the practical utility of the probes as a solid state kit for the onsite detection of NAC explosives.


 Please wait while we load your content...
Please wait while we load your content...