Blue-emitting cationic iridium(iii) complexes featuring pyridylpyrimidine ligands and their use in sky-blue electroluminescent devices†‡
Abstract
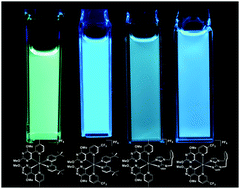
The synthesis and structural and photophysical characterisation of four novel, cationic iridium(III) complexes are reported. These complexes were designed to emit in the blue region of the visible spectrum without employing sp2 carbon–fluorine bonds, which have been shown to be electrochemically unstable. Two different C∧N (where C∧N is a bidentate cyclometalating ligand possessing a nitrogen–carbon chelate) ligands [5-(4-methylpyridin-2-yl)-2,4-dimethoxypyrimidine (Mepypyrm) and 5-(5-(trifluoromethyl)pyridine-2-yl)-2,4-dimethoxypyrimidine (CF3pypyrm)] combine electron-withdrawing pyrimidyl nitrogen atoms (in a para relationship with respect to the metal) with methoxy groups in a meta relationship with respect to the metal, which both inductively withdraw electron density from the metal centre, stabilizing the highest occupied molecular orbital. The result is highly efficient (ΦPL = 73–81%) green to blue (λPL = 446–515 nm) emission for complexes 1–4 in MeCN solution. Complex 1 exhibits a broad, unstructured charge transfer (CT) emission profile, while complexes 2–4 exhibit structured, vibronic emission profiles. Density Functional Theory (DFT) calculations corroborate these findings with spin density calculations predicting a T1 state that is metal-to-ligand and ligand-to-ligand (C∧N to N∧N) charge transfer (3MLCT/3LLCT) in nature for complex 1, while complexes 2–4 are predicted to exhibit ligand-centred (3LC) states with spin density localised exclusively on the C∧N ligands. These complexes were used as emitters in sky-blue and blue-green light-emitting electrochemical cells (LEECs). The bluest of these devices (CIE: 0.23, 0.39) is among the bluest reported for any iridium-based LEEC. It is noteworthy that although the electroluminescence intensity decreases rapidly with time (t1/2 = 0.1–20 min), as is typical of blue-green LEECs, for devices L1, L3 and L4 we have observed for the first time that this decay occurs without an accompanying red-shift in the CIE coordinates over time, implying that the emitter does not undergo any chemical degradation process in the non-doped zones of the device.


 Please wait while we load your content...
Please wait while we load your content...