Dual fluorescent zwitterionic organogels of a quinoxalinone derivative using cation–anion detection keys†
Abstract
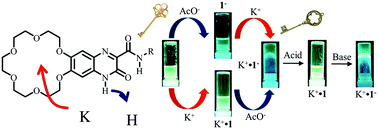
An [18]crown-6 unit was introduced into a cation–anion dual-ion-sensing quinoxalinone derivative (1) as a new fluorescent molecule for successive cation (K+) and anion (F− and CH3COO−) sensing in CH3CN. High anion-sensing abilities for F− and AcO− were observed at the hydrogen-bonded acidic N–H proton of the positively charged K+-capturing 1 at the [18]crown-6 site due to electrostatic cation–anion interactions. On the other hand, the acidic N–H proton of lactam tautomer 1 strongly recognized basic AcO− or F− anions via N–H⋯AcO− or F− hydrogen-bonding interactions, and further AcO− or F− additions facilitated the deprotonation reaction, forming anionic 1−. Anionic 1− showed a much higher K+-sensing ability at the [18]crown-6 site than neutral 1 through effective cation–anion electrostatic interactions. Interestingly, the electrostatically stabilized zwitterionic K+·1− formed fluorescent organogels in CH3CN, acetone, and THF; the organogels underwent reversible transformation between a blue fluorescent organogel and green fluorescent sol by the addition of trifluoroacetic acid (gel → sol) and trimethylamine (sol → gel). Both K+ and AcO− (or F−) ions acted as the key ions in the fluorescent organogel formation.


 Please wait while we load your content...
Please wait while we load your content...