Color-tunable fluorescent nanoparticles encapsulating trialkylsilyl-substituted pyrene liquids†
Abstract
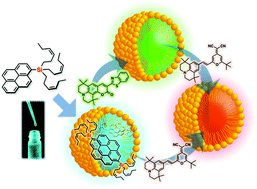
The development of emissive liquid dyes containing trialkylsilyl groups, and their use in the preparation of fluorescent organic nanoparticles (FONs) are described. A series of trialkylsilyl-substituted pyrenes is synthesized as oils, whose viscosity depends on the nature of the alkyl groups. The neat compounds show intense excimer emission with maxima of around 460–480 nm and quantum yields of 0.5–0.7. The low viscosity (η = 162 cP) of 2-tri((Z)-3-hexen-1-yl)silylpyrene (1) allows the preparation of spherical nanoparticles (NP1) in the presence of 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC) and cholesterol. NP1 shows a hydrodynamic diameter of 161 ± 48 nm and maintains an excimer emission similar to that of neat 1. NP1 in water spontaneously absorbs dopant dyes into its liquid core, and simultaneous doping with two dyes is also possible. When using green-emissive C545T and red-emissive DCJTB dyes as dopants, efficient Förster resonance energy transfer (FRET) occurs from the pyrene excimer to the dyes. Extensive emission color tuning is thus achieved as evident from a plot of the emission colors of doped and undoped NP1 on the CIE 1931 xy chromaticity diagram. These results demonstrate the efficacy of the present design strategy for color-tunable FONs that do not require premixing of dopant dyes.


 Please wait while we load your content...
Please wait while we load your content...