Superbenzene-bridged bis(permethyl-β-cyclodextrin) as a convenient and effective probe for trinitrophenol exploder†
Abstract
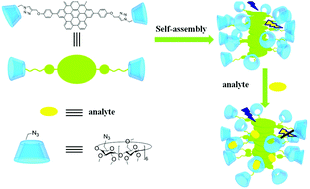
A novel planar C2 symmetrical superbenzene-bridged bis(permethyl-β-CD) was synthesized by grafting two permethyl-β-CD units onto a coronene core. Its self-assembly and luminescent behaviors could be smartly controlled by adjusting the polarity of the environment. Significantly, the resultant self-assembly showed specific fluorescence responses to exploders, especially 2,4,6-trinitrophenol, over various common aromatic compounds, which could be readily distinguished either in solution or on the test paper, and the detection limit of self-assembly towards 2,4,6-trinitrophenol could reach the ppb level. This finding would enable the self-assembly as a convenient and highly efficient fluorescence sensor for the detection of exploders.


 Please wait while we load your content...
Please wait while we load your content...