Transfection-capable polycationic nanovectors which include PEGylated-cyclodextrin structural units: a new synthesis pathway†
Abstract
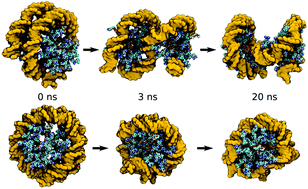
Efficient tools are still being searched for to substitute the viral vectors in nucleic acid delivery applications. One of the most severe constraints in producing them is related to the strict reproducibility of their molecular characteristics, which is ensured through the synthesis. In this work, we report an original route to obtain polycationic nanoentities with low variability, which are able to act as cooperating carriers for dsDNA complexation and transport. The carriers are synthesized by rigorous conjugation of β-cyclodextrin (β-CD) with precise ratios of 2 kDa branched poly(ethyleneimine) (b-PEI) and 0.75 kDa poly(ethylene glycol) (PEG). Low cytotoxicity was the key parameter of the carrier design, besides the highest possible transfection ability, and both of these features were proven by HeLa cell culture assays. A reporter gene which induces the expression of green fluorescent protein (GFP), inserted in a plasmid, was used to perform the necessary quantitative measurements. In silico molecular modelling guided the carrier design and confirmed the functional mimicry of histones in the tight and compact nucleosome-like spiral packaging of dsDNA. The carrier molecules, synthesized with high reproducibility, are expected to be feasible for application in gene transfection.
- This article is part of the themed collection: Celebrating Excellence in Research: Women of Materials Science


 Please wait while we load your content...
Please wait while we load your content...