Thermoresponsive star-like γ-substituted poly(caprolactone)s for micellar drug delivery†
Abstract
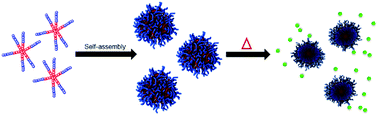
Temperature responsive drug carriers are attractive due to their ability to provide controlled release of the encapsulated cargo based on the use of external stimuli. In this work, 4- and 6-arm thermoresponsive star-like block copolymers were synthesized through the ring-opening polymerization of γ-substituted ε-caprolactone monomers γ-2-[2-(2-methoxyethoxy)ethoxy]ethoxy-ε-caprolactone (MEEECL) and γ-ethoxy-ε-caprolactone (ECL) using pentaerythritol and myo-inositol as multifunctional initiators. These amphiphilic block copolymers were shown to self-assemble into micelles and were characterized in terms of their feasibility as drug carriers. Both polymers were shown to be thermodynamically stable and demonstrated temperature responsivity in a desirable range for drug delivery, with lower critical solution temperatures of 39.4 °C and 39.8 °C for the 4- and 6-arm polymers, respectively. It was shown that the 6-arm star polymer had a higher drug loading capability and better stability in vitro, allowing it to function as a better vehicle for drug delivery in cytotoxicity experiments. These star polymers show promise as drug carriers due to their biocompatibility, biodegradability, and temperature controlled release of doxorubicin.


 Please wait while we load your content...
Please wait while we load your content...