Electrocatalytic reduction of CO2 to CO with 100% faradaic efficiency by using pyrolyzed zeolitic imidazolate frameworks supported on carbon nanotube networks†
Abstract
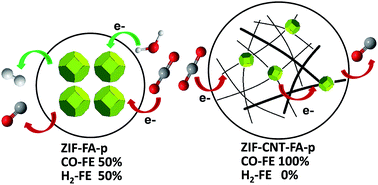
Electrochemical reduction of CO2 has received much attention because of its potential in converting atmospheric CO2 into commodity products. One of the key challenges in electrochemical CO2 reduction is the competing hydrogen evolution reaction (HER) at reduction electrode potentials. In this work, we used a hybrid material composed of pyrolyzed zeolitic imidazolate frameworks (ZIFs) and multi-walled carbon nanotubes (MWCNTs) to selectively catalyze electrochemical reduction of CO2 to CO in aqueous solution with close to 100% faradaic efficiency and a current density up to 7.7 mA cm−2 at an overpotential of 740 mV. The MWCNT support is crucial to achieving superior selectivity, thanks to enhanced electron transport on the MWCNT network and expedited CO2 transport in the mesoporous structure constructed by the MWCNTs. Moreover, doping Fe into the hybrid further reduces the overpotential of CO2 reduction to 440 mV with a current density of 2 mA cm−2 and a CO faradaic efficiency of 97%. This work highlights the importance of optimizing electron and mass transport in turning moderate catalysts into superior ones with high activity and selectivity for CO2 reduction.


 Please wait while we load your content...
Please wait while we load your content...