Competitive reactions during synthesis of zinc aluminum layered double hydroxides by thermal hydrolysis of urea†
Abstract
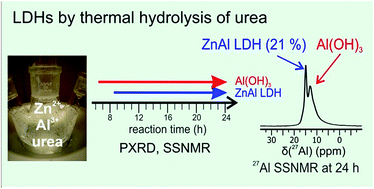
Homogeneous precipitation by thermal hydrolysis of urea (“The urea method”) is preferred for the preparation of pure and highly crystalline layered double hydroxides (LDHs). However, our recent study revealed large concentrations of amorphous aluminum hydroxide (AOH) in several zinc(II) aluminum(III) LDHs (ZnAl-LDHs) prepared by this method. The origin of this AOH, the nature of an elusive zinc-rich phase, and whether phase pure LDHs can be obtained by the urea method remained unanswered [J. Phys. Chem. C., 2015, 119, 27695–27707]. Therefore, a series of ZnAl-LDHs were prepared by the urea method at four different reaction times (7, 12, 16, and 24 h) and characterized by bulk (PXRD, TEM, and elemental analysis) and local techniques (27Al SSNMR, FT-IR, and Raman spectroscopies) in combination with a time-resolved synchrotron PXRD study of the reaction mixture. The products obtained are a mixture of hydroxylated phases: an ill-defined aluminum hydroxide with similarities to gibbsite precipitates at 7 h, which is followed by a competitive formation of a crystalline ZnAl-LDH (≈8 h) and a poorly crystalline hydrozincite during the later stages of the reaction. Their relative concentrations vary depending on the synthesis conditions, e.g., reaction time, urea/metal ratio, metal source, and hydrothermal treatment post synthesis, with less than half of the total Al3+ content incorporated in ZnAl-LDHs according to solid state 27Al NMR. Thus, the preparation of highly crystalline and pure LDHs remains a challenge. Furthermore, these impurities will have a significant impact on application studies using LDHs prepared by the urea method.


 Please wait while we load your content...
Please wait while we load your content...