Metal–organic framework-derived CoSe2/(NiCo)Se2 box-in-box hollow nanocubes with enhanced electrochemical properties for sodium-ion storage and hydrogen evolution†
Abstract
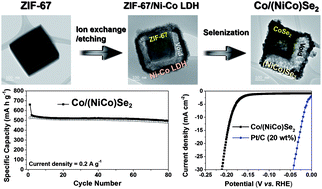
Multishell structured metal selenide nanocubes, namely, Co/(NiCo)Se2 box-in-box structures with different shell compositions, were successfully synthesized by applying zeolitic imidazolate framework-67 (ZIF-67) as a template. This strategy involved the fabrication of cube-shaped ZIF-67/Ni–Co layered double hydroxides with a yolk–shell structure and then transformation into Co/(NiCo)Se2 with a box-in-box structure by a selenization process under Ar/H2 conditions. During the selenization step, hollow structured CoSe2 cores were generated by Ostwald ripening, resulting in the formation of Co/(NiCo)Se2 with a box-in-box structure composed of an inner CoSe2 shell and an outer (NiCo)Se2 shell. Due to the synergetic effect of the unique structure and multicomponent selenide composition, the Co/(NiCo)Se2 with the box-in-box structure offered excellent dual functionality as both an anode for sodium ion batteries (SIBs) and an electrocatalyst for the hydrogen evolution reaction (HER). Electrochemical tests on the Co/(NiCo)Se2 with the box-in-box structure demonstrated a low Tafel slope (39.8 mV dec−1) and excellent stability. In addition, it delivered a high specific capacity of 497 mA h g−1 after 80 cycles, with a current density of 0.2 A g−1 and excellent cycling stability as an anode material for SIBs.


 Please wait while we load your content...
Please wait while we load your content...