Ag–Ni core–shell nanowires with superior electrocatalytic activity for alkaline hydrogen evolution reaction†
Abstract
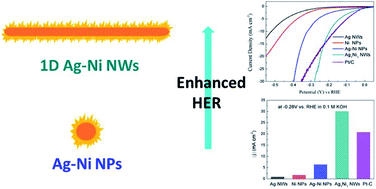
One-dimensional Ag–Ni core–shell nanowires were successfully synthesized by a simple two-step method, in which Ni shells grow epitaxially at the surface of silver nanowires (Ag NWs) to form a unique core–shell structure. The thickness of Ni shells can be finely controlled from 50 to 150 nm by adjusting the initial Ni to Ag atomic ratios. The electrocatalytic performance of the Ag–Ni core–shell NWs can be adjusted by tuning their compositions, and the optimized Ag to Ni atomic ratio is 1 : 1. The Ag–Ni core–shell nanowires exhibit superior electrocatalytic activity to pure Ni nanoparticles and Ag NWs, showing a strong synergistic effect toward alkaline hydrogen evolution reaction (HER). A morphological effect was demonstrated by comparing with Ag–Ni core–shell nanoparticles. The enhanced HER activity of Ag–Ni core–shell NWs can be attributed to the synergetic effect between Ag and Ni, the larger number of surface active sites and the fast charge transport. This approach provides a reasonable attempt for designing and developing 1D electrocatalysts based on Ag nanowires.


 Please wait while we load your content...
Please wait while we load your content...