How does TiF4 affect the decomposition of MgH2 and its complex variants? – An XPS investigation†
Abstract
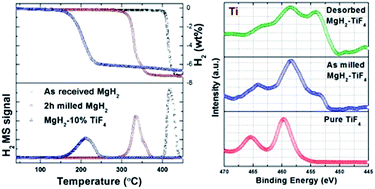
Magnesium hydride and its complex variants, i.e. Mg(AlH4)2 and Mg(BH4)2 are known for their high hydrogen capacity. The theoretical capacities of these three are 7.6 wt%, 9.3 wt% and 14.4 wt%, respectively. In spite of having very attractive H2 capacities, their high operating temperature, i.e. more than 300 °C and sluggish kinetics are big issues to be solved before these can be realized for a practical hydrogen storage system. There are several efforts devoted to reduce the working temperature and enhance the sorption kinetics using various additives. Ti-based additives have always been interesting contenders in enhancing the kinetics as well as altering thermodynamics thus reducing the working temperature for various hydrides. Recently, TiF4 has shown superior catalytic activity on the decomposition of Mg(AlH4)2. This motivates us to study its effect on the decomposition of all the above-mentioned Mg-based hydrides. It is observed that the addition of 10 wt% TiF4 to the above materials greatly influences the decomposition temperature. The decomposition temperature for all three reactions is shifted to the lower temperature side. The oxidation state of the catalyst surface has been investigated using the XPS technique and then the detailed mechanism associated with this improvement is proposed herein.


 Please wait while we load your content...
Please wait while we load your content...