Naphthalene diimide-based small molecule acceptors for organic solar cells†
Abstract
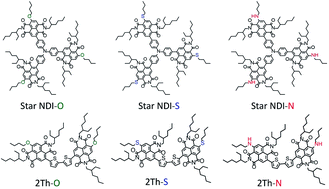
This work introduces six novel naphthalene diimide (NDI) molecular acceptors for evaluation in organic solar cells based on two different chemical architectures: a star-shaped structure with a triarylamine core flanked by three NDI moieties and a linear molecule composed of a bithiophene bridge between two NDI moieties. For each molecular structure, three different side chains are examined, with alkyl chains linked to the NDI core either through oxygen, sulfur, or nitrogen substituents. Both the chemical structure and the side-chain heteroatom substitution were found to influence the optoelectronic and photovoltaic properties of these molecular acceptors. Organic solar cells were fabricated with each acceptor, utilizing PBDTTT-EFT (also known as PTB7-Th) as the donor material in inverted bulk-heterojunction devices. Nitrogen was observed to lower the solar cell performance for these acceptors by significantly decreasing the short circuit current density (JSC), while sulfur increased the JSC and, in the star configuration, led to the highest power conversion efficiency (PCE) of 2.8% – which is amongst the highest for any molecular NDI-based acceptor to date. Grazing incidence wide-angle X-ray scattering (GIWAXS) measurements of the star-shaped materials showed the side-chain substitutional atom significantly alters the material's packing configuration in neat films, with films blended with PBDTTT-EFT showing features characteristic of the neat donor and acceptor materials, indicating that the small molecules do not disrupt the packing of PBDTTT-EFT (and vice versa). Resonant soft X-ray scattering (R-SoXS) measurements indicate the PBDTTT-EFT:star-shaped acceptor blends are not subject to coarse phase-separation, with the average domain size for all three star-shaped acceptor blends typically being less than 100 nm. This is confirmed by similar topography for blended films in AFM images amongst the three acceptors. Photoluminescence (PL) quenching measurements, however, found large differences in PL quenching efficiency which were attributed to differences in the driving force for charge transfer, with the nitrogen substituted compound showing the lowest PL quenching and the sulfur replacement showing the highest.


 Please wait while we load your content...
Please wait while we load your content...