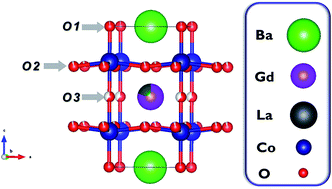
Relating defect chemistry and electronic transport in the double perovskite Ba1−xGd0.8La0.2+xCo2O6−δ (BGLC)
Abstract
Rare earth double perovskites comprise a class of functional oxides with interesting physiochemical properties both for low- and high-temperature applications. However, little can be found relating electrical properties with equilibrium thermodynamics of non-stoichiometry and defects. In the present work, a comprehensive and generally applicable defect chemical model is developed to form the link between the defect chemistry and electronic structure of partially substituted BGLC (Ba1−xGd0.8La0.2+xCo2O6−δ, 0 ≤ x ≤ 0.5). The equilibrium oxygen content of 4 different compositions is determined as a function of pO2 and temperature by thermogravimetric analysis, and combined with defect chemical modelling to obtain defect concentrations and thermodynamic parameters. Oxidation enthalpies determined by TG-DSC become increasingly exothermic (−50 to −120 kJ mol−1) with increased temperature and oxygen non-stoichiometry for all compositions, in excellent agreement with the thermodynamic parameters obtained from the defect chemical model. All compositions display high electrical conductivities (500 to 1000 S cm−1) with shallow pO2-dependencies and small and positive Seebeck coefficients (3 to 15 μV K−1), indicating high degree of degeneracy of the electronic charge carriers. The complex electrical properties of BGLC at elevated temperatures is rationalized by a two-band conduction model where highly mobile p-type charge carriers are transported within the valence band, whereas less mobile “n-type” charge carriers are located in narrow Co 3d band.


 Please wait while we load your content...
Please wait while we load your content...