A novel discharge–charge mechanism of a S–P2S5 composite electrode without electrolytes in all-solid-state Li/S batteries†
Abstract
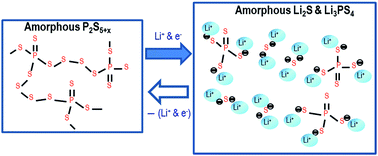
All-solid-state Li/S cells with high safety and high capacity were fabricated using a sulfur composite electrode prepared by mechanically milling S, P2S5 and a conductive additive (Ketjen black). The cells with 50 wt% sulfur content in the composite electrode showed a high reversible capacity of 942 mA h (g-sulfur)−1 at a constant current density of 0.64 mA cm−2 (0.1C). The discharge–charge mechanism of the high-capacity sulfur composite electrode without electrolytes was investigated. XRD and NMR measurements showed that amorphous P2S5+x species, where sulfur chains bridged phosphorus atoms, were produced in the as-milled composite electrode. Mixing of the amorphous P2S5+x and Ketjen black in the submicron order was indicated from the FE-SEM observation and EDX mapping of the electrode. XRD and TEM measurements of the sulfur electrodes before and after the discharge–charge processes indicated that the compounds in the electrodes remained in the amorphous state during these processes. XPS measurement showed that cleavages and associations of the disulfide bonds occurred in the amorphous compounds during the discharge–charge processes. A novel discharge–charge mechanism with an atomic-level dispersion of a sulfur redox part in an ion conductive part was proposed for the high-capacity sulfur electrode.


 Please wait while we load your content...
Please wait while we load your content...