Wet chemical synthesis of apatite-based waste forms – A novel room temperature method for the immobilization of radioactive iodine†
Abstract
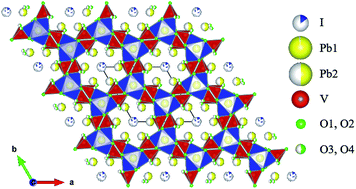
Iodine isotopes make up ∼0.69% of the fission products of 235U slow neutron fission, i.e. ∼360 g iodine per metric ton of 235U. The currently proposed technology for the removal of iodine from reprocessing plant off-gas is to pass it through a caustic scrubber (using ∼1–2 M NaOH) to form NaI, NaIO3 and possibly NaOI, or to pass it through Ag-containing solid sorbents to form chemisorbed AgI. Since iodine is not amenable to conventional vitrification routes because of its poor incorporation into borosilicate glass chemistry and high volatilization at temperatures >500 °C, considerable effort is being made to develop alternative waste forms for the immobilization of iodine at low temperatures (<100 °C). In this paper, a novel wet chemical synthesis method has been described for immobilizing radioactive iodine obtained from caustic scrubber solutions and silver solid sorbents into the apatite, Pb10(VO4)6I2, at room temperature. The uptake of iodine for the synthesized waste form has been shown to be 94.6%. In addition, investigation into the formation of solid solutions in the systems, Pb(10−x)Cax(VO4)6I2 and Pb10(VO4)(6−y)(PO4)yI2, by the proposed method has been discussed. The study provides an easy pathway to design novel waste forms for the immobilization of large volumes of radioactive iodine at room temperature and a methodology to potentially synthesize new compositions of iodide-containing apatites.


 Please wait while we load your content...
Please wait while we load your content...