A facile corrosion approach to the synthesis of highly active CoOx water oxidation catalysts†
Abstract
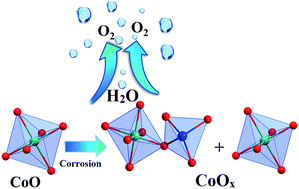
Ultra-small rock salt cobalt monoxide (CoO) nanoparticles were synthesized and subjected to partial oxidation (‘corrosion’) with ceric ammonium nitrate (CAN) to form mixed-valence CoOx (1 < x < 2) water oxidation catalysts. Spectroscopic, microscopic and analytical methods evidenced a structural reformation of cubic CoO to active CoOx with a spinel structure. The superior water oxidation activity of CoOx has been established in electrochemical water oxidation under alkaline conditions. Electrochemical water oxidation with CoOx was recorded at a considerably low overpotential of merely 325 mV at a current density of 10 mA cm−2 in comparison to 370 mV for CoO. Transformation of both octahedral CoII and CoIII sites into amorphous Co(OH)2–CoOOH is the key to high electrochemical activity while the presence of a higher amount of octahedral CoIII sites in CoOx is imperative for an efficient oxygen evolution process.


 Please wait while we load your content...
Please wait while we load your content...