Hydrogen weakens interlayer bonding in layered transition metal sulfide Fe1+xS†
Abstract
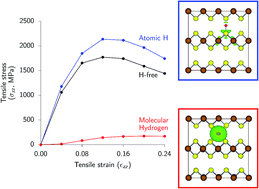
The presence of interlaminar interstitial defects like hydrogen affects the mechanical properties of van der Waals-bonded layered materials such as transition metal chalcogenides. While the embrittling effect of hydrogen is well understood in metals, the impact of hydrogen defects on the mechanical behavior of layered chalcogenides remained unexplored. In this article, we use density functional calculations to reveal the influence of different hydrogen point defects on important mechanical metrics, including binding energies, elastic moduli and tensile and shear strengths of a prototypical ionic layered material, mackinawite, Fe1+xS. We find that one of the low-energy hydrogen defect structures, interlaminar molecular H2 interstitials, severely degrades the strength of inter-layer van der Waals interactions in the mackinawite crystal. This leads to a significant (over 80%) degradation in the mechanical properties of the mackinawite crystal and enables facile interlayer sliding and exfoliation. This finding suggests the mechanisms for cathodic exfoliation of transition metal chalcogenides like Fe1+xS, and presents a plausible mechanism for the poor protectiveness of layered passive films like mackinawite that undergo failure by spalling or delamination.


 Please wait while we load your content...
Please wait while we load your content...