Structure and stability of CaH2 surfaces: on the possibility of electron-rich surfaces in metal hydrides for catalysis†
Abstract
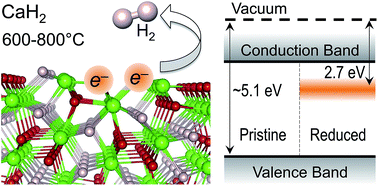
Structure, thermodynamic stability, and electronic properties of CaH2 surfaces in (001), (110), and (111) crystallographic orientations are investigated using ab initio modeling. We show that stoichiometric surfaces terminated with a hydrogen atomic plane are the most energetically favorable and discuss properties of hydrogen vacancies (VH) at these surfaces. The average calculated work function of the most stable pristine surfaces (∼5.2 eV) is in agreement with experimental data for powder samples. Neutral hydrogen vacancies host localized electrons and induce defect states in the band gap, thereby shifting the effective work function to much lower values of ∼2.7 eV. Surface VH are predicted to aggregate into dimers and form electron-rich centers (e−)Ca2+(e−) stable to over 800 K. These results suggest that hydrogen-deficient surfaces of CaH2 can host a large concentration of localized electrons and, thus, give rise to new catalytic functionalities involving electron transfer between the surface, catalysts supported on it, and reacting species.


 Please wait while we load your content...
Please wait while we load your content...