Mixed-valence NaSb3O7 support toward improved electrocatalytic performance in the oxygen-reduction reaction†
Abstract
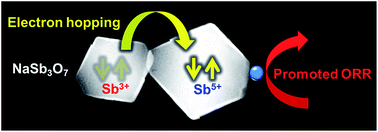
Nanocrystals of sodium antimony oxide, NaSb3O7 (pyrochlore structure, a = 1.030 nm), act as an efficient catalyst support for the electrocatalytic oxygen-reduction reaction (ORR) in acidic media. The NaSb3O7 nanocrystals (edge length ∼ 150 nm) were synthesized by hydrothermal decomposition of SbCl5 in aqueous solution of NaOH. The NaSb3O7 nanocrystals were then decorated with Pt nanoparticles by chemical reduction of H2PtCl6 in water to yield an ORR catalyst, Pt/NaSb3O7. The Pt/NaSb3O7 exhibited higher ORR performance than the state-of-the-art Pt/TiO2- or Pt/C catalysts in terms of the +40 mV higher half-wave reduction potential and the retained electrochemical surface area than the Pt/TiO2 after 10 000-times repeated ORR in an acidic electrolyte. Unlike NaSb3O7, Pt-decorated Sb2O5 (Pt/Sb2O5) was much less active than the Pt/TiO2 or Pt/C. The enhanced ORR activity of the Pt/NaSb3O7 may be attributed to the promoted electron hopping between the Sb3+ and Sb5+ ions in mixed-valence Na1+(Sb3+Sb25+)O7, which is inhibited in single-valence Sb25+O5.


 Please wait while we load your content...
Please wait while we load your content...